Fatty = superficial tension?Slimous = small surface tension?
Author:Institute of Physics of the Ch Time:2022.09.17
After a smooth heavy rain, after
The lotus pond restored the calmness of the past.
Lying on the lotus leaves,
Enjoy the sun in the afternoon.
A gust of wind blows,
The lotus leaves shake slightly,
Water droplets roll on the leaf surface ...
Behind the seemingly harmonious picture,
It is actually a grievance of hundreds of millions of years between molecules.
It is also a gap that it is difficult to eliminate the world!
all of these,
Everyone must start with a familiar term,
Surface Tension.
· Part 1: Force, not a simple pull and pull ·
Friends who are familiar with physics must be familiar with the strength around them: gravity, elasticity, support, Kulun power ... Every time you mention a force, most of them can emerge in the mind. You push me, you pull me.
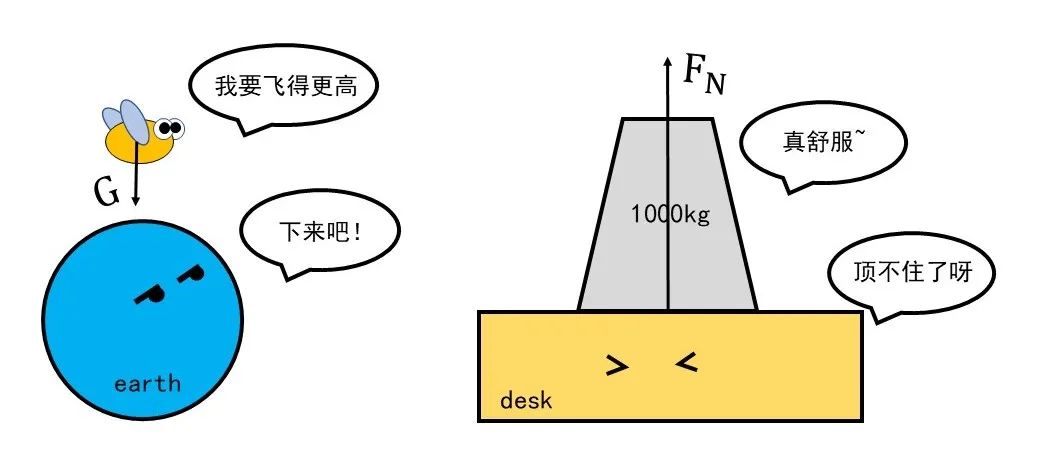
Figure 1 Gravity and Supporting Power
Although this understanding is intuitive, it lacks a little more in -depth perspective, and there is no way to truly understand what the surface tension is. then what should we do? Let's take a look at the energy corresponding to the power (specifically the potential energy). Taking gravity as an example, everyone may have heard of gravity potential, that is, the energy contained in an object near a certain earth is at a certain height. More intuitively, if the ground is zero, the energy that the object can release to the ground under the action of gravity is gravity potential.
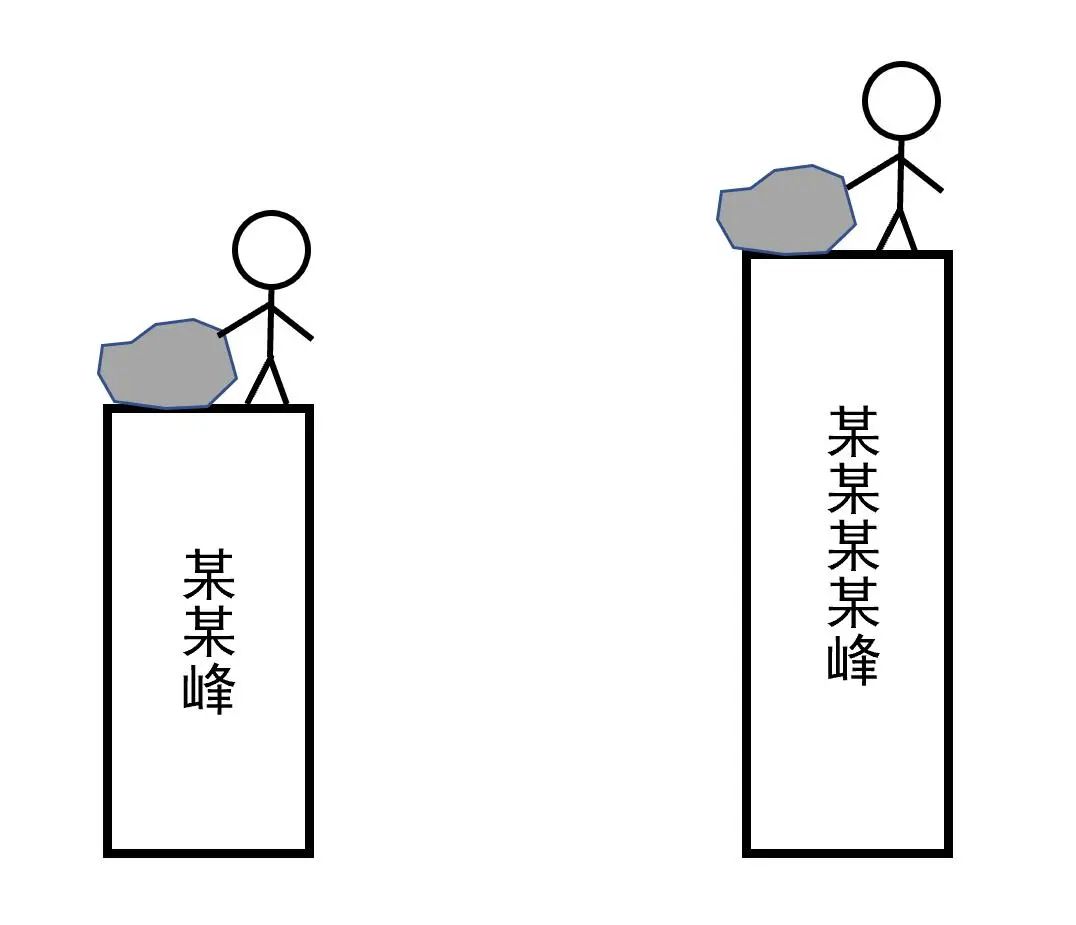
Figure 2 Comparison of gravity potential energy
As shown in Figure 2, if Xiaobian pushes the same stone from a relatively low peak and a taller and higher peak, which gravity of the release is great? Obviously, it is even greater from a higher peak. So how much is it?
If the gravity changes with height, that is, the gravity of the stone is a constant G, then the answer is:
Among them, DL is the height difference between the two peaks. With a little deformation, we get it
This shows that gravity is the change of gravity potential energy brought about by the height of each increase! Similarly, the spring elasticity is the changes in the elastic potential energy brought by the length of the unit. The inter -molecular force is the changes in the system potential of the system of the molecular distance. Power can be understood like this.

At this time, Xiaobian asked elegantly: So what is the surface tension?
Readers answered unrequitedly: The surface energy changes brought about by the area of the unit area on the surface!
Xiaobian asked after applauding: What is the surface? What direction is the surface tension?
The reader was silent, and the look at the expectation to the editor ...
· Part 2: tension, but it's just blowing a bubble ·
In fact, the problems mentioned above can be seen intuitively from the outline. We have all been in the concept of doing work. The units of work and energy are Jiao Er J. The distance between the force (unit of N) and the force of the object to move in the direction of the object (the unit is m), that is,: 1 j = 1 n · m. Then, in addition to N can also write J/m, the energy changes caused by 1m movement.

However, there is an outline of the physical quantity of J/M, which represents the energy contained in the area of each unit. This physical quantity is better than the surface. Surface can be a concept involving the role of molecules, and we need to know from the micro.
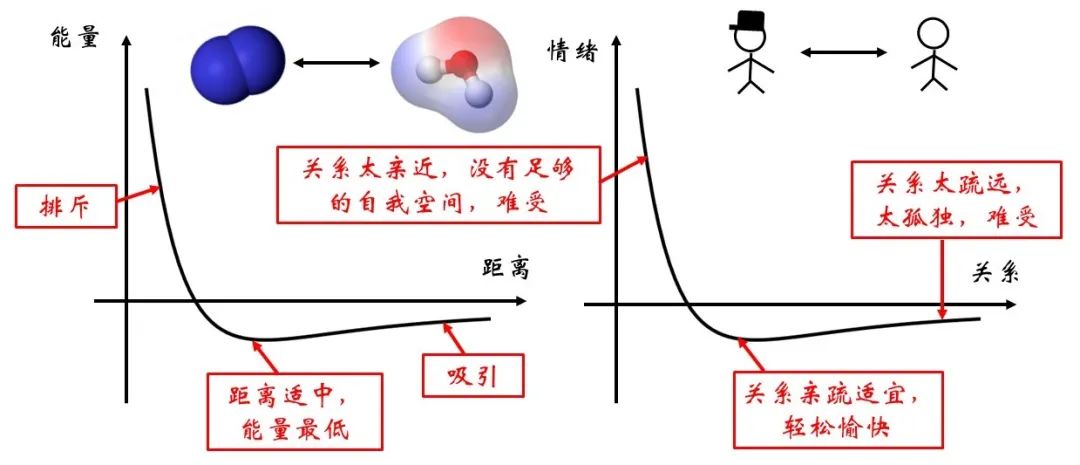
Figure 3 icon of the potential energy between nitrogen and water molecules
There are a variety of molecules in the micro world, such as nitrogen and water molecules shown in the figure above. The potential energy of the system between molecules is related to the distance between the molecule. This relationship is just like the relationship between two people, far away is not good. If the distance is too close and there is no self -space between each other, the emotions will be unstable, so they will have a mutually rejection; if the distance is too far, everyone will miss each other and attract each other. When the distance between each other is just right, it corresponds to the lowest point of energy and is relatively stable.
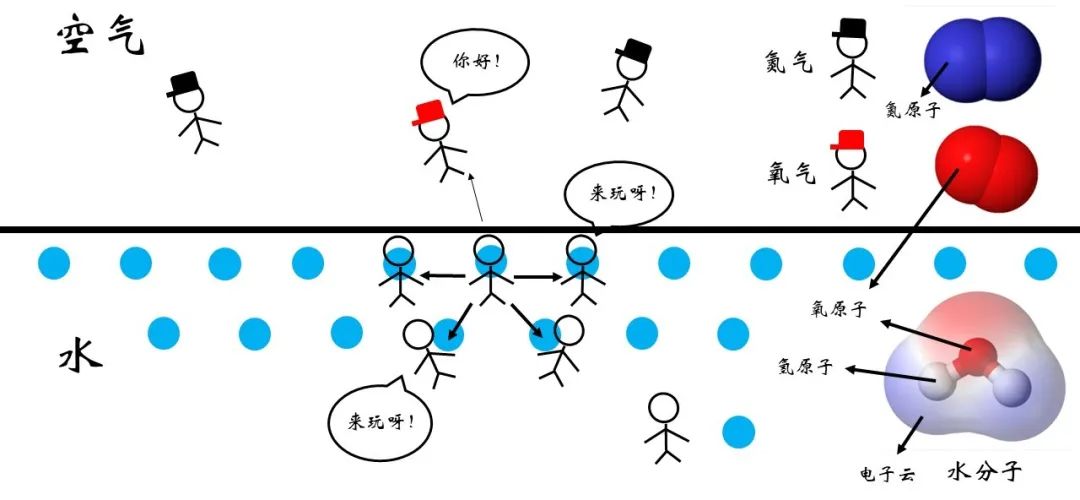
Figure 4 Water-air interface signal
However, the degree of stability of this relationship is related to the type of molecules between the two sides. As shown in Figure 4, for the interface of water-air, the types of molecules around the surface are richer. In addition to other water molecules, there are also molecules such as oxygen and nitrogen in the air.
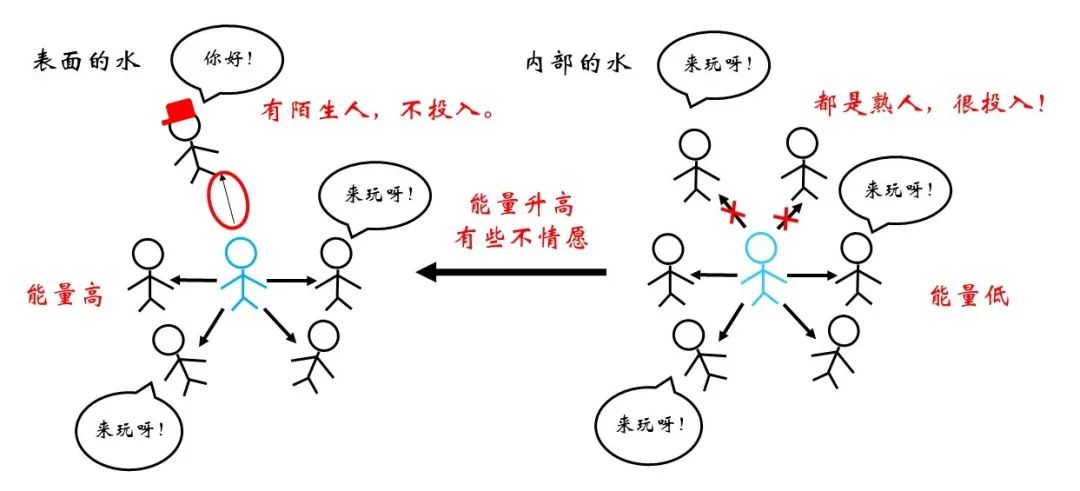
Figure 5 Internal and surface water comparison
As the saying goes, "things are gathered in class, people are divided into groups", and more similar relationships between similar molecules are often more harmonious, while the relationship between different types of molecules is relatively alienated. The minimum potential curve between similar molecules is lower in physical performance. Therefore, for a water molecule that exists inside, if you come to the surface, you need to disconnect some of the relationship with similar molecules, and the role of "rusty" air molecules is obviously reluctant! This is reflected in the rise of energy. So, what happens to the surface from the inside? It was when it was blowing bubbles.
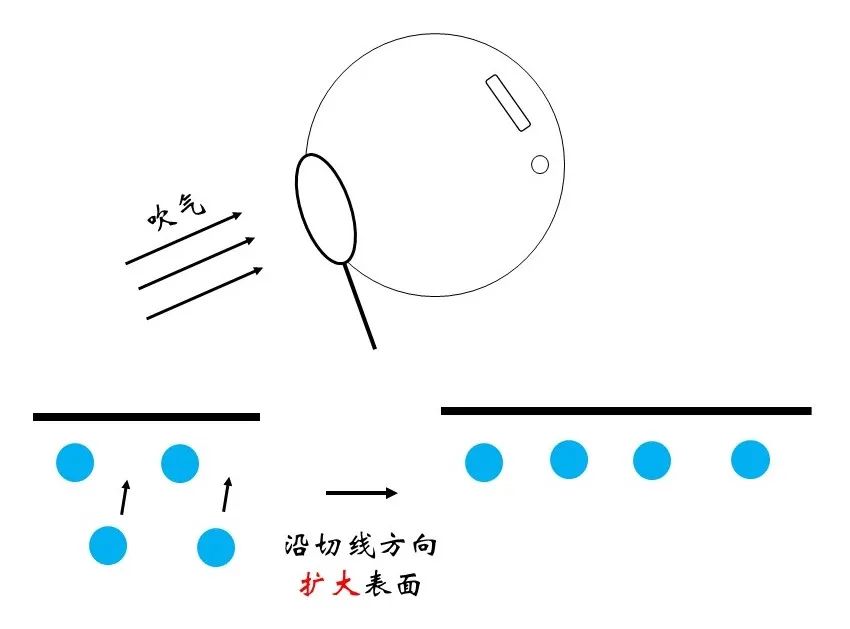
Figure 6 Silent
Dip some soapy water with rings, the volume of soapy water is basically constant. When we blow into the air to make the surface area of bubbles greater, the thickness of natural bubbles will become thinner. Further explanation is that more water molecules will come from the inside to the surface, and this process is reluctant to internal molecules, so it will bring energy to increase. So, what is the increase in energy from the area of each expansion unit on the surface? This value is exactly than the surface.
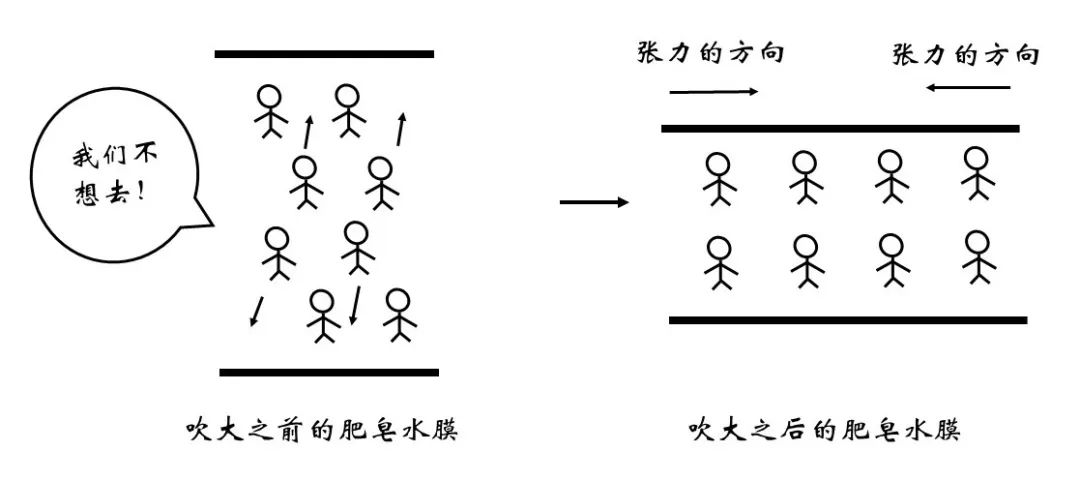
Figure 7 Surface increase with soap bubbles becomes thinner
We once mentioned that the unit of the surface energy is J/M. It is more intuitive to understand this physical quantity from the perspective of strength. We often call the surface tension than the surface. The J/M = N/M is not a unit N, so the surface tension represents the surface force on the surface of the unit length. In the experiments shown in the figure below, the soap and water film was pulled up with the pull F, and the surface tension was among them. The two on the denominator came from the surface of the front and back.
Figure 8 surface tension experiment schematic diagram | Picture derived from [1]
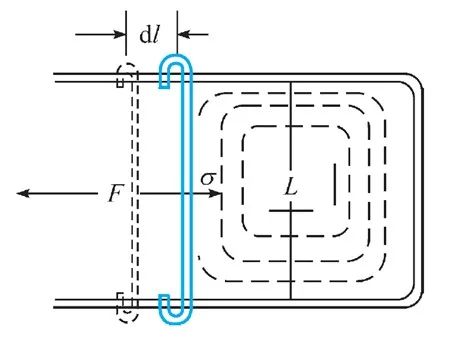
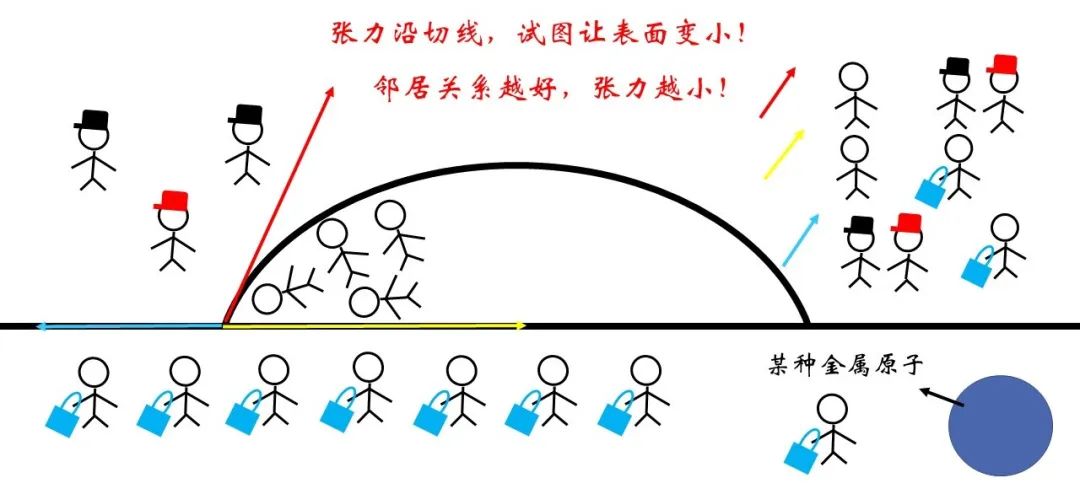
So what is the direction of the surface tension? We have the same type of comparison and elasticity. Simple review is not difficult to find that if the surface tension is understood as the increase in energy caused by the shape of the unit area, the corresponding force should point to the direction where the energy is reduced. For the surface tension, it is naturally the direction of the surface shrinking along the line.
Figure 9 The direction of the surface tension indicates
· Part 3: Pursue spherical, tension and gravity dispute ·
Before this part starts, the first question is raised: What shape will the droplets present under weight loss?
With a little memories, readers who have seen space lectures should think of the spherical water droplets suspended in space.

Figure 10 Sales screen screenshot screenshot screen

That's right, the droplets in the weightless environment are the spherical shape, because at a certain volume, the surface area of the sphere is the minimum, so the total surface of the spherical liquid droplet is the lowest. So, in the case of gravity?
Figure 11 The trend of the surface molecules entered the interior
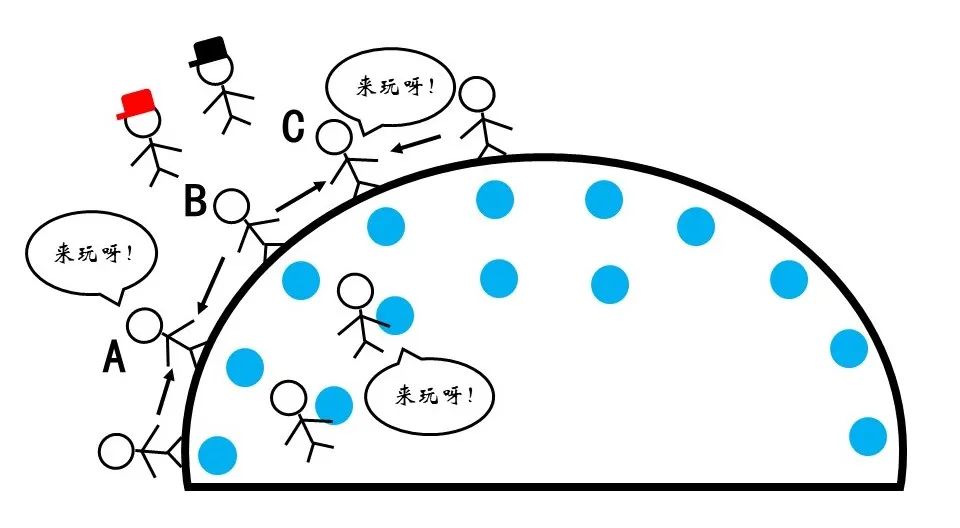
On the surface of the droplet, each molecule will be subject to the surface tension of the tangent. The effect is equivalent to each molecule (such as A and C) that is pulling around (such as B). The joint force of the surrounding surface molecules is pointing to the interior, and there is a tendency to enter the inside. The role of this tension makes the droplets maintain a state of ascending the spherical shape as much as possible, that is, it has as small as possible. However, gravity plays another role.
Figure 12 The role of gravity
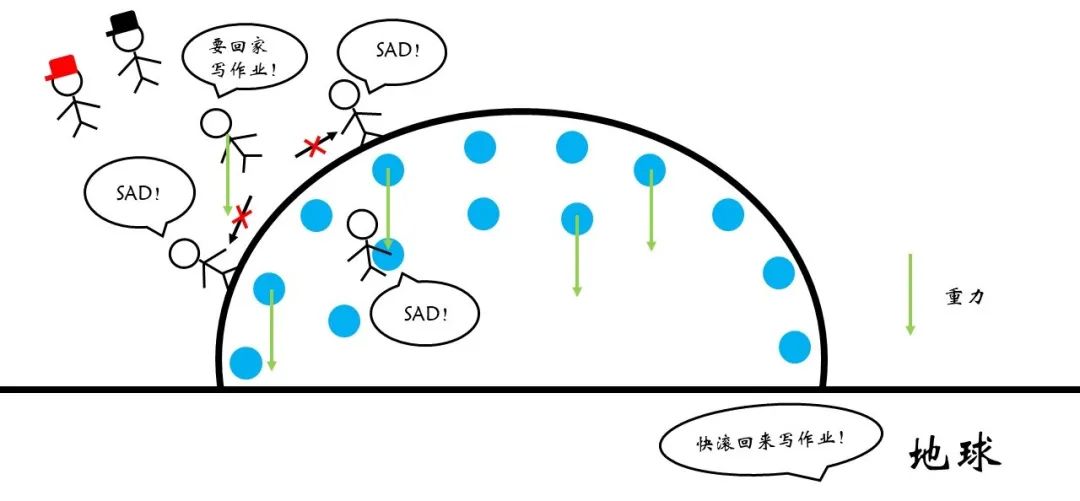
The role of gravity hopes that each water molecules are as low as possible. Obviously, the shape of approximate spherical shape is not the lowest of gravity potential energy. Therefore, there is competition between surface tension and gravity.
Figure 13 competition between gravity and tension
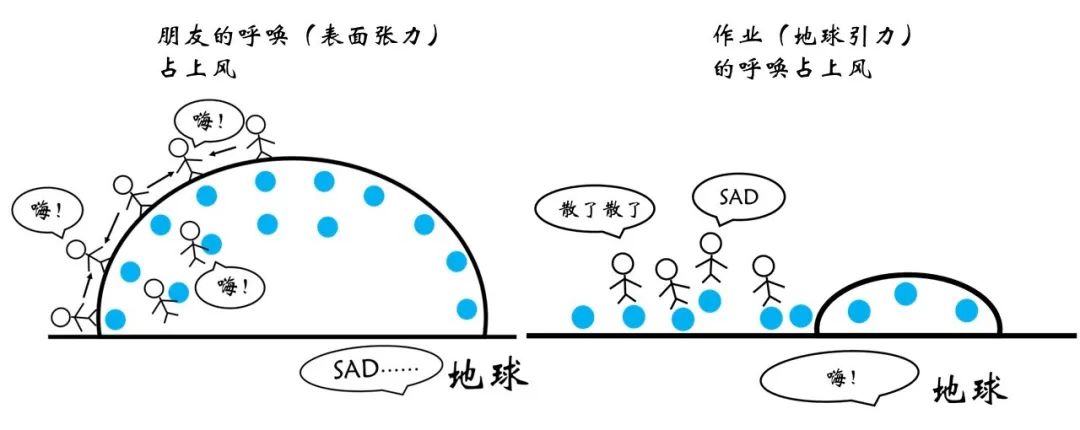
It is not difficult to imagine that if the tension is strong, the droplets will maintain the shape of the near -shaped shape without collapsed. If the tension is weak and cannot compete with the gravity, the droplets will be broken. Back to the problem of blowing bubbles, the reason why it is generally dipped in soapy water instead of pure water is because the surface tension of pure water is small, which is not enough to compete with the gravity of the liquid film. However, in space, gravity no longer acts, and the water film can exist steadily!
Figure 14 water film in space

Further, it involves a problem: What is the size of the surface tension of different substances? In terms of definity, we can introduce the concept of affinity, which reflects the affinity relationship between the material on both sides of the interface. If the material on both sides is very close, then the interior and the surface are actually not much different. After all, everyone can fit, so the surface tension is relatively small. Conversely, if the material relationship between the two sides is very alienated, the surface tension will be great. And this is the root cause of the infiltration phenomenon.
· Part 4: Infiltration, love and hatred between molecules ·
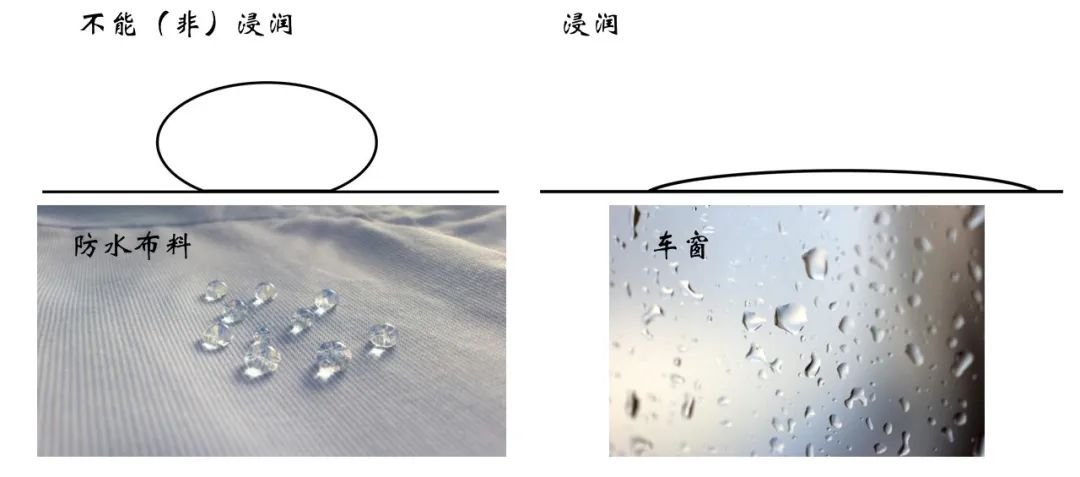
Infiltration, the word may be more unfamiliar with everyone, but the corresponding physical phenomenon is very common. For example, the waterproof clothes shown in the figure below and the water droplets on the bus window are non -infiltrated and infiltrated representative scenes.
Figure 15 The phenomenon of infiltration and non -invasion in life
Observing the above scene carefully, we found that there are not only two substances (to be precise, because the air has complex components), but three types -one is a solid, a liquid, and environment for the base Gas in. Therefore, the interfaces involved in the infiltration problem we are facing are not one, but three types of two or two.
Figure 16 Sales of material relationships
Naturally, the surface tension will exist on any interface, and its size is related to the relative affinity relationship between the species on both sides, and the direction of the direction is directed to the interface to shrink the interface. The final state needs to balance the weight of these three forces.
Figure 17 competition at the junction of the three interfaces
For infiltration, the relationship between liquid and solid matter is very good. It can be considered that the surface tension at the interface is small enough to be ignored. In this way, the horizontal component of red and blue force needs to be balanced, and the shape of the droplets tends to flatter. Generally, the angle of the tension and the liquid solid interface of the gas liquid interface is generally called contact angle, and the contact angle of the hydrophilic surface with significant infiltration phenomena is small.
Figure 18 Infiltrate the micro signal
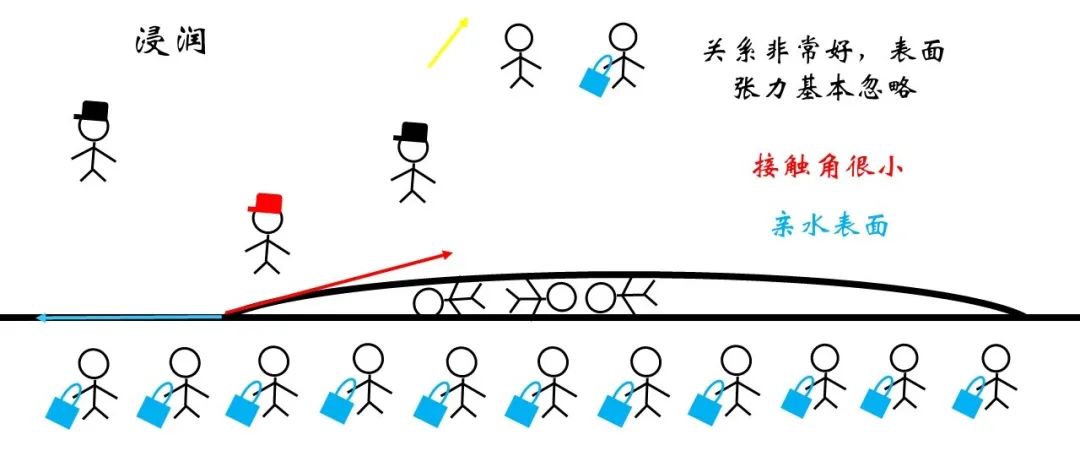
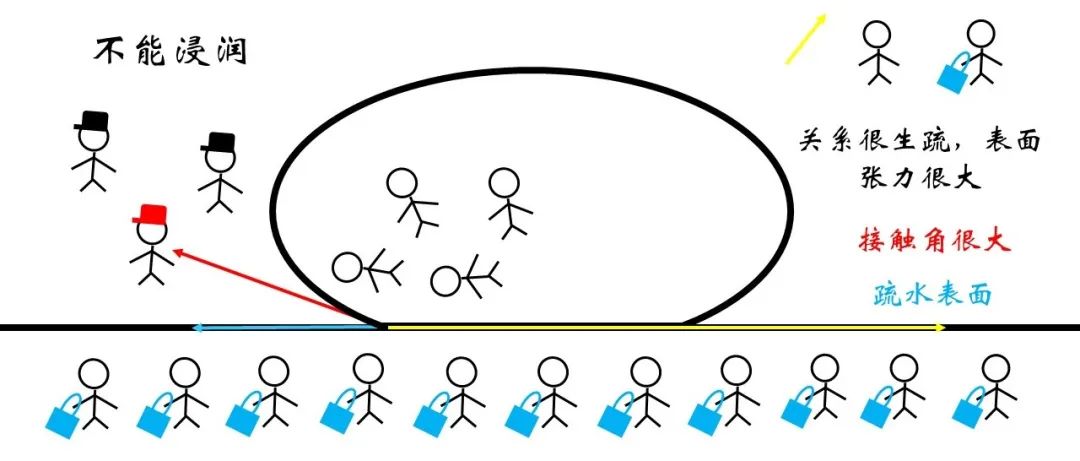
Conversely, the surface tension of the solid surface may be very large for the surface of the hydrophobic surface, so that the contact angle can only reach balance only when the contact angle is reached blunt angle or even approaching 180 °. At this time, the phenomenon is called non -infiltration or cannot be infiltrated. This surface is called ultra -hydrophobic surface because of high hydrophobicity.
Figure 19 non -invasive micro signal
Speaking of which, some readers may have guessed that the surface of the lotus leaf is a natural ultra -hydrophobic substance, so the water is close to the spherical shape on the surface of the lotus leaf.
Figure 20 The ultra -hydrophobic phenomenon in nature
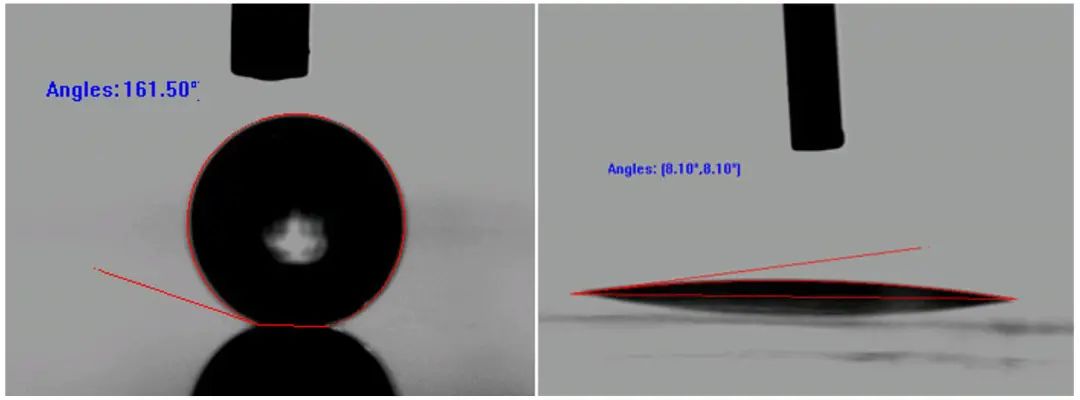
Not only in nature, scientists also prepare more significant hydrophilic or super -water surfaces in the laboratory, such as the extreme non -infiltration and infiltration phenomenon in the figure below.
Figure 21 Artificial Water Rehabilitation/Super Live Surface | Pictures derived from [2] //
Night fell,
A night wind blows,
The lotus leaf shakes sharply,
Several water drops roll off in the pond,
Great -ripples.
A water puppet passes across the water,
Only a few rushing,
It disappeared in the white moonlight.
Where do it know,
I support my body,
It is a magical surface tension ...
Reference materials:
[1] Zhu Zhiang, Ruan Wenjuan. Physical Chemistry. 6th Edition [M]. Science Press.
[2] Ultra-hydrophobic/Super-water surface modification-for various plastics
Edit: Cloud Kaiye
- END -
Gather the matrix resources to accurately help the enterprise to relieve the rescue!Guiyang High -tech Zone helping enterprises crossing difficulties to promote steady economic development

Help industrial enterprises apply for various types of funds at all levels, carry ...
Why is the background of the spacemen's outspoken image?

On September 1st, Chen Dong and Liu Yang, the Shenzhou 14 of my country, successfu...