Oxidation reaction application
Author:Middle school chemical root ca Time:2022.08.27
High school chemistry synchronous lecture
Lecture: Teacher Zong Wei
Shaanxi Province's first provincial high school chemical backbone teachers and teaching experts, outstanding counselors of national chemistry competitions, senior chemical teachers in middle school, once taught in Xi'an No. 1 Middle School, Xi'an E -Science and Technology University Affiliated High School, West Institute of Technology Cultural Tuition School, and Xi'an Huanggang Cultural Tuition School
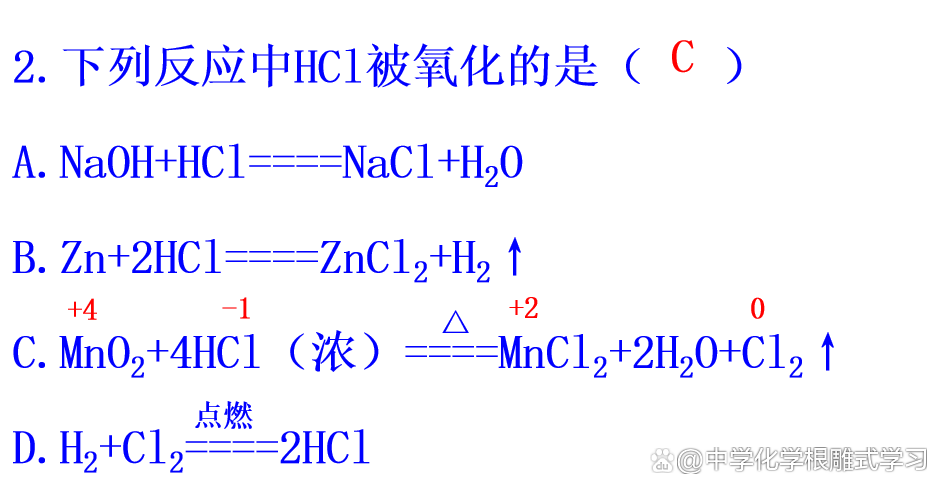
3. The following narrative about the oxidation reaction is correct (d)
A. All elements in the reaction have changed
B. Lost electrical substances, the elemental price contained in the element is reduced
C. The reaction of the substance by the oxidation itself
D. There must be electronic transfer (gain or loss or offset)
Basic laws of oxidation reaction reaction
1. Conservation law
Total number of e -electron loss in the restoring agent = total number of electrons or decreases at the total number of electrons or oxidizers = the total number of rising videos of the original agent.
2. Law of strength
In an oxidation reaction, the oxidation and reduction of each material are: oxidation: oxidant> oxidation product
Restore: Restore> Restore product
Application: Compare the strength of oxidation or restoration between substances, and judging the reaction between particles must be strong.
3. Rules
A variety of oxidants meet with a reducing agent. The strong oxidation is first of all electronics. A variety of reducing agents meet with a oxidant. The strong reduction of the electrons is first oxidized.
For example, when the chlorine gas is connected to the FeBR2 solution, the reducing FE2 +> BR, if the amount of chlorine is insufficient, oxidize Fe2 +; when the chlorine gas is connected to the Fei2 solution, the restoration I-> Fe2 +. Essence
Application: Determine the order of substances.
4. Law
(1) Law of height: The maximum price state is only oxidation, the lowest price state is only reduction, and the intermediate price state is both aerobic and restored.
Application: Judge the oxidation and restoration of elements or substances.
(2) Law of the price state: When the oxidation reaction occurs between the atoms of different prices of the same element, the high -priced + low -cost state -→ middle price state; that is, "only closer, not cross".
Application: Determine the price of elements in generics.
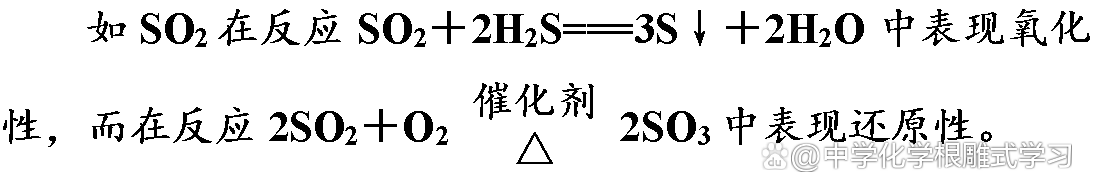


(1) The strength of oxidation and reduction is only related to the difficulty of loss of electrons at the atom of the element, and it has nothing to do with the number of gains and losses electrons. The stronger the electronic ability, the stronger its oxidation; the stronger the electronic ability, the stronger the restoration.
(2) Whether the substance is oxidized or redundant is related to the nature of matter. Some substances only have oxidation, such as F2; some substances only have reduction, such as K, CA, etc.; Some substances are both oxidized and restored.

(3) Metal cations are generally oxidized, but low -cost cations such as FE2 + are both oxidized and restored.
(4) No oxidation reaction occurs between the same element adjacent price state.



In conclusion
1. The reducing agent is a reactor that loses electrons (or electrons to deviation). The component of the element contained in the reaction increases. The restoring agent is restored, and it is oxidized when the reaction is oxidized. The oxidant corresponds to it.
2. When the element is at the highest price, it is only oxidized, the lowest price is only reduced, and the aerobicity and restoration of the middle price are both aerobic.
3. In a given oxidation reaction, oxidation: oxidant> oxidation product; reduction: reducing agent> reducing product.
4. N (oxidant) × The number of atoms of the price reduction element in the chemical formula × the reduction value of the composite price = n (reducing agent) × the number of atoms of the atom in the chemical formula × the increase in the value of the combined price.
JPEG "Webkit-Playsinline>



- END -
"Science and Technology Innovation China" technical roadshow Nankai special event was successfully held

On August 5, the Science and Technology Innovation China technical roadshow was su...
From electricity to light, the Chinese chip industry is moving towards the light | Jiazi Guangnian

The Chinese core opportunity in the Moore eraAuthor | XiaoxianEdit | chestnutAfter...