Sale national, emergency recall!Many people have
Author:Voice of Zhejiang Time:2022.07.15
Source: China Pharmaceuticals, State Drug Administration
The copyright belongs to the original author, if there is any infringement, please contact it in time
On July 14, the official website of the State Drug Administration issued a notice on 19 batches of medicines that did not meet the requirements. Details are as follows:
State Drug Administration
A notice on 19 batches of medicines do not meet the requirements
(No. 32, 2022)
After inspection by 10 pharmaceutical inspection institutions such as Hubei Pharmaceutical Supervision and Inspection and Research Institute, 19 batches of drugs such as acetylcysteine injections produced by Ruiyang Pharmaceutical Co., Ltd. were not in line with regulations. The relevant situation is notified as follows:
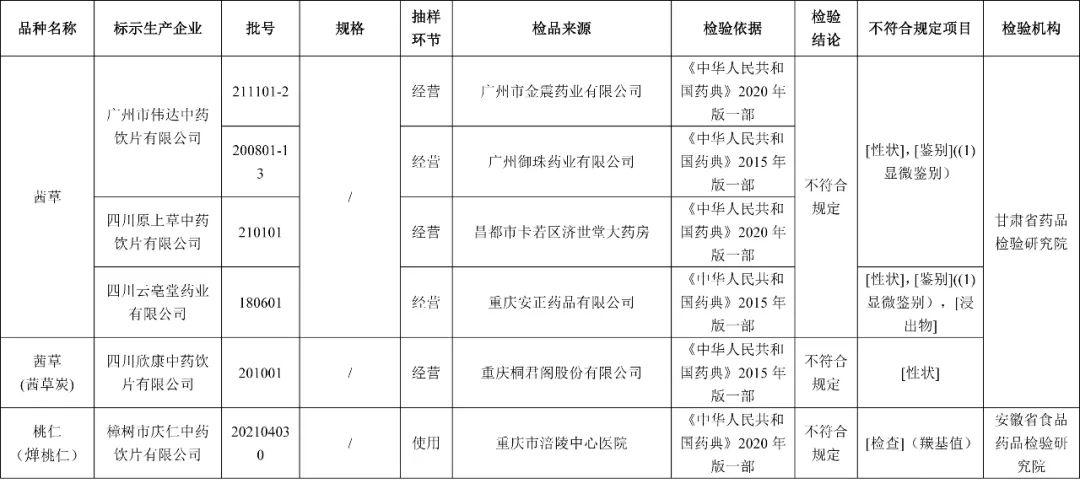
I. The inspection by the Liaoning Provincial Pharmaceutical Inspection and Inspection Institute, marked that a batch of 1 batch produced by Shanghai Diran City Pharmaceutical Co., Ltd. relies on erythromycin particles and does not meet the regulations, and does not meet the requirements of the specified project as the content measurement.
After inspection by the Hubei Provincial Institute of Drug Administration, the two batches of acetylcysteine injection produced by Ruiyang Pharmaceutical Co., Ltd. did not meet the regulations and did not meet the specified projects as hydrogen sulfide.
The two batches of Longze Xiong Bile Capsules produced by Tonghua Zhongsheng Pharmaceutical Co., Ltd. did not meet the requirements and did not meet the requirements of the specified projects and collapse time limit.
After inspection by the Food and Drug Inspection and Research Institute of Shandong Province, a batch of ginseng Jianpi Pills produced by Tianjin Sino -Singapore Pharmaceutical Group Co., Ltd. Daren Pharmaceutical Factory did not meet the regulations, and did not meet the requirements of the specified projects as the difference.
After inspection by the Food and Drug Inspection and Research Institute of Tibet Autonomous Region, a batch of fifteen -flavored Black Pharmaceutical Pills produced by the Tibetan Pharmaceutical Factory of Tibet did not meet the regulations, and it did not meet the requirements of the specified projects as heavy differences and microorganisms.
After inspection by the Food and Drug Inspection Institute of Henan Province, the two batches of Ai leaves produced by Harbin Songshantang Pharmaceutical Co., Ltd. and Sichuan Jiuwei Pavilion Traditional Chinese Medicine Crimination Co., Ltd. do not meet the requirements, and the projects are not in line with regulations.
After inspection by the Sichuan Pharmaceutical Inspection and Research Institute (Sichuan Medical Device Inspection Center), a batch of Sichuan Achyranthes bulls produced by Hubei Minatai Pharmaceutical Co., Ltd. did not meet the regulations and did not meet the requirements of the specified projects as traits.
After inspection by the China Food and Drug Inspection Research Institute, the three batches of chrysanthemums produced by Jiangsu Donglian Pharmaceutical Co., Ltd., Anhui Wan Sheng Traditional Chinese Medicine Drinking Co., Ltd., and Hunan Songling Traditional Chinese Medicine Drinking Co., Ltd. did not meet the requirements, and did not meet the regulations. The projects are banned from pesticide residues.
After inspection by the Gansu Provincial Academy of Pharmaceutical Inspection and Research, it is marked as the 4 batches of Qiancao produced by Guangzhou Weida Traditional Chinese Medicine Drinking Co., Ltd., Sichuan Yuancao Traditional Chinese Medicine Drinking Co., Ltd., and Sichuan Yunbatang Pharmaceutical Co., Ltd. The project includes traits, micro -identification, and immersion; 1 batch of Qiancao (Qiancao charcoal) produced by Sichuan Xinkang Traditional Chinese Medicine Drinking Piece Co., Ltd. does not meet the requirements and does not meet the specified projects as traits.
After inspection by the Institute of Food and Drug Inspection of Anhui Province, a batch of peach kernels (燀 燀 燀) produced by Zhangshu City Qingren Traditional Chinese Medicine Drinking Co., Ltd. did not meet the regulations and did not meet the requirements of the regulations as the base value.
2. For the above -mentioned do not meet the prescribed drugs, the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales, recall, and recall, and conduct investigations and rectification on reasons for not compliance.
3. The State Drug Administration requires relevant provincial drug supervision and management departments to organize investigations on suspected illegal acts existing above -mentioned enterprises and units in accordance with the "Drug Administration Law of the People's Republic of China", and publicize the results of the investigation and punishment in accordance with regulations.
announce.
State Drug Administration


(Click to enlarge it to view)

Tips that do not meet the specified projects
I. The content measurement item means the content of the raw materials and the effective ingredients in the preparations using the specified test method. Generally, chemical, instruments or biological measurement methods can be adopted.
Second, the hydrogen sulfide solution status acetylcysteine (the performance of acetylcysteine's effect on the effect of acetylcysteine is dependent on its cymbal group that can make the glycoprotein polypeptide chain in the sputum break) The formation of unstable falling off is irritating to the respiratory system.
Third, the appearance, odor, smell, solubility, and physical constant, etc., reflect the quality characteristics of the medicine to a certain extent. Traditional Chinese medicines do not meet the regulations, and may involve the deviation of the species of medicinal materials, defects, and improper storage of the processing process.
Fourth, the time limit of the collapse refers to the collapse of the index solid system under the prescribed conditions. Any preparation that stipulates the degree of dissolving, release, fusion time limit or dispersing uniformity, will no longer be checked.
Fifth, differences in weight (difference in volume) are indicators that reflect the uniformity of drugs, and are one of the important parameters to ensure accurate administration.
6. Microbial limits are microbial control requirements for drug preparations that are not directly entering the human environment. Due to the slightly lower risk of drugs for such drug preparations, a certain number of microorganisms can be allowed, but some conditional pathogenic bacteria shall not be detected. Microbial limits are divided into two parts: counting testing and controlling bacterial examination.
7. The disable the amount of pesticide residues reflects the disabled pesticide disabled pesticide in Chinese medicinal materials or slices.
8. Identification items are mainly used to distinguish between drug characteristics. The means include micro -identification, spectral identification, etc. The thin layer chromatography is a commonly used method.
Nine, immersion items can reflect the content of the inner components of Chinese medicinal materials and slices, and the irregularity of the origin, growth period, harvest season, processing method, and processing process of traditional Chinese medicinal materials and slices may cause their immersion content to do not meet the regulations.X. The base value reflects the amount of substances such as the iliac -based compounds produced by the fat containing Chinese medicinal materials and slices, which may be related to improper storage.
Data-nickName = "The Voice of Zhejiang" Data-ALIAS = "ZHEJIANGZHISHING" Data-SIGNATURE = "What you hear is happening. The Voice of Zhejiang, synchronize with the news." Data-from = "0" />
- END -
Shu Dao Group's "Hand in Hand" Chengdu Air Railway International Transport Port Multi -type Multi -type Transportation Services arrives in the "last mile"

Cover reporter Liu Qiufeng Photography ReportOn the morning of June 16th, at the ...
The active changes in my country's economic operations have increased: industrial production has been significantly accelerated from decreasing to foreign trade imports and exports
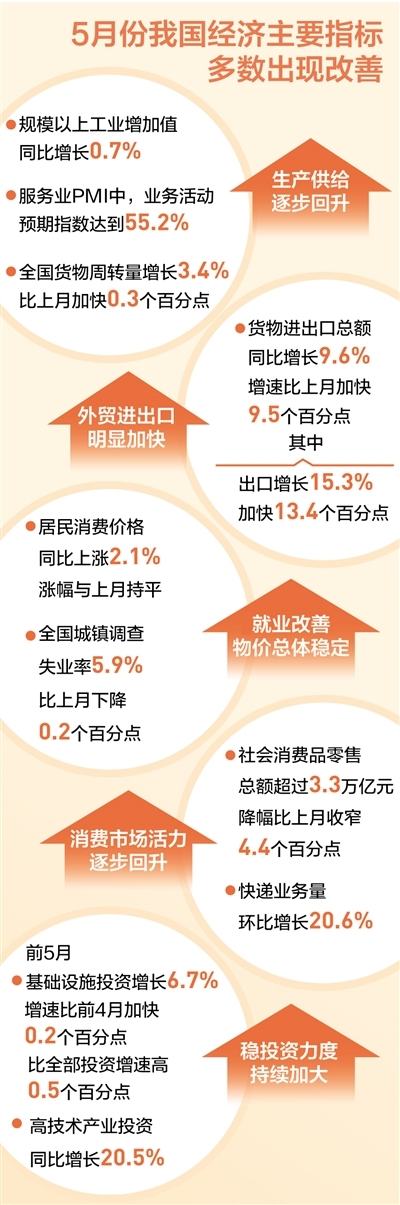
In May, industrial production has increased from decline, foreign trade imports an...