Hengrui Pharmaceutical's new AR inhibitor is approved by patients with prostate cancer to usher in a new choice
Author:Zhongxin Jingwei Time:2022.07.02
Zhongxin Jingwei, July 2nd. On June 29th, Hengrui Pharmaceutical Announcement announced that it was recently received the "Drug Registration Certificate" approved by the State Drug Administration. (Commodity name: Eric) Treatment of metastatic hormone -sensitive prostate cancer (MHSPC) patients used for high tumor load. At this point, Hengrui Pharmaceutical's innovative drugs have increased to 11 domestic listing.
The results of the Chart research based on the approval show that compared with standard therapy, the Ryoviramine combined withrogens deprivation therapy (ADT) can significantly extend the patient's overall survival period (OS) Risks are 42%and 54%, respectively. The listing of Revilunamide is expected to provide a new superior effect treatment plan for patients with prostate cancer in China.
CHART research is a multi -center, random, and controlling phase III clinical trial. The study aims to explore the efficacy and safety of the treatment and safety of the Reviloamine combined ADT comparison standard treatment in patients with high tumor load MHSPC. The research results show that the combination of Rivilu amine combined with ADT can significantly extend the overall survival period (OS) of patients with high tumor load MHSPC and significantly reduce the risk of disease progress or death of patients. A total of 654 patients were studied in the study, and 90.4%of the domestic patients accounted for 90.4%, which met the current status of diagnosis and treatment of Chinese patients. Based on the assessment of the Independent Judgment Committee (IRC), compared with the control group, River amine reduced 42%of the death risk of patients and the risk of 54%of the imaging progress. The 2 -year survival rate of patients with Ryoviramine medication was 81.6%(70.3%in the control group, Figure 1), and 79.2%of the 2 -year -old imaging progress rate (49.4%in the control group, Figure 2). Compared with the control group, compared to the control group The 2 -year survival rate and no imaging progress rate have improved significantly; in terms of safety, the Ryoviraman group did not treat the death of related adverse events (TRE).
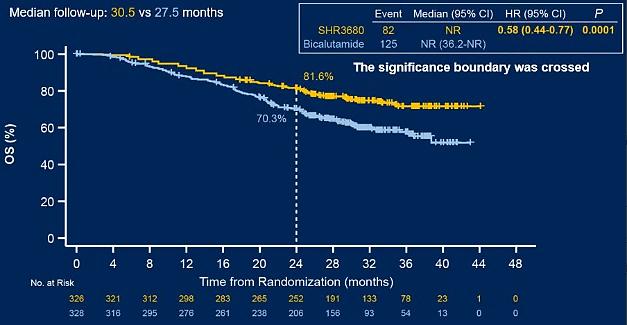
figure 1
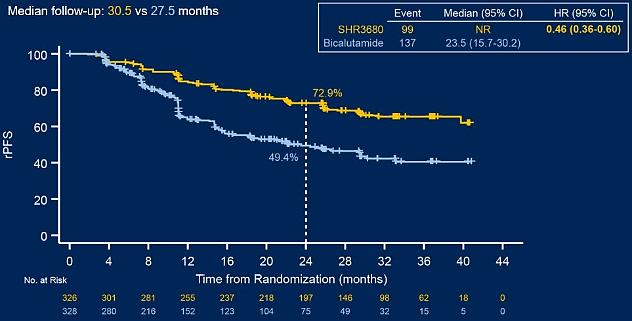
figure 2
The results of the above research have been unveiled in the form of verbal reports at the Annual Conference of the United States Clinical Oncology (ASCO) in 2022 this year, showing an important progress of Chinese urinary tumor innovation drugs on the international stage. Based on the results of this research, Riviramine has obtained the "Guidelines for the diagnosis and treatment of prostate cancer (2022)" of the China Clinical Oncology Society (CSCO) ", providing new treatment options for Chinese pre -prostate cancer patients.
According to reports, prostate cancer is the second common malignant tumor in men worldwide, and it is also the fifth cancer species of mortality. The incidence of prostate cancer in China is 15.6/100,000, and it is increasing year by year. Most patients have metastasized during the initial diagnosis, and the prognosis is not good. The growth of prostate cancer cells hasrogen dependencies. Due to the continuous activation of therogen receptor (AR) signal pathway, even if the patient receives the treatment of potential therapy, it will inevitably develop to resist prostate cancer and more likely to metastasize metastasis The five -year survival rate of metastatic prostate cancer is less than 30%. Miscipline hormone -sensitive prostate cancer (MHSPC) is mainly based on new endocrine therapy. The second -generation AR inhibitor can effectively delay the time to enter metastatic removal resistance prostate cancer and extend the total survival period of patients. At present, there have been two new AR inhibitors that have been approved by the world's MHSPC indications, and only one domestic was approved in 2020, so the treatment choices of domestic patients are still very limited.
According to the data, Riviluamine is a new type of AR inhibitor developed with independent intellectual property development developed by Hengrui Pharmaceutical. This product has made important innovations in the structure of the drug molecular. Significantly reduced permeability compared with the listing of similar products on the market, reducing central nervous toxicity, as well as more optimized pharmacokinetic characteristics.
In September and October 2021, the indications of the metastatic hormone sensitivity prostate (MHSPC) patients with high tumor load (MHSPC) were used to be included in the list of breakthrough treatment centers and priority by the Drug Review Center of the State Drug Administration, respectively. Review approval procedure. The approved listing will effectively promote the application of the new AR inhibitor in China, so that more patients with prostate cancer can benefit from standardized treatment.
Another phase III study of Revilunamide, that is, the international multi -center, random, and control phase III clinical study that treats high -risk prostate cancer during the treatment of high -risk prostate cancer is currently being promoted in an orderly manner. (Zhongxin Jingwei APP)
- END -
Stopping the "Banning Wildlife Transaction Announcement" to make Zhaoyan's new drug fall?Guojin Securities is expected to rise in the second half of the year

Due to the acquisition of the two experimental animal model companies to complete ...
The first batch of "Park City Demonstration Zone Construction Opportunity List" was released!Article 1070 supply and demand information, an estimated investment of 580 billion yuan

How to build a park city demonstration zone with new development concepts, how to ...