Chu Pu Beauty Calls recall the product but refuses to return the goods.
Author:Burning news Time:2022.09.16
Future Network, Beijing, September 16th (Li Tianqi reporter Wang Junwei) Recently, the future reporter learned from the black cat complaint platform that the platform released the August corporate complaint to handle the red and black list. Due to the release of product recalls in hidden safety hazards, he refused to return the goods for users, and was collectively complained by consumers. It ranked first in the Black Cat complaints in August.

Picture source: Screenshot of Black Cat Complaint Platform
The official announcement recalls the refusal to return, and the well -known beauty instrument has caused controversy
In the future, reporters from the Internet were informed that on July 29, the Defective Product Management Center of the State Administration of Market Supervision and Administration (hereinafter referred to as defective Product Management Center) issued a recall notice because there is a risk of scald skin, and the beauty instrument brand Chu Pu in the new foundation of the Chinese dealer in China (Shenzhen) Technology Co., Ltd. (hereinafter referred to as Xinzhixin Technology) actively recalls 182215 first -generation first -generation STOP EYE model household radio frequency beauty instruments.
However, many consumers "do not buy it" for the recall of the beginning of the prescription.
"My appeal is to return the goods. At the beginning of the Pu, he denied that the quality problem was not accepted." Zhejiang consumer Wang Jia (pseudonym) complained to the future network reporter that in June 2020, she purchased this Stop Eye beauty instrument, in the place, in the past It is often hot during use.
"The defective product management center issued a notice that I was determined that it was not my problem at all." Wang Jia said that I asked Chupu Company to return the goods on the grounds of product quality. Active initiation of the risk that may occur for the use of consumers' improper use, it is not a quality problem, "so it is not returned.

Photo source: Consumers provide
Can't repair it? How to protect rights to buy defective products?
In fact, Wang Jia's experience is not a case, and Shanghai consumers Li Yue (pseudonym) also encountered the same thing.
"The merchant introduces the beauty instrument for three minutes in one eye, but it will not work in less than three minutes." Li Yue complained to the future network reporter, "I used to think it was normal, and the result was that it had a problem with the quality problem. I asked for a refund. I asked Chupu how to ensure that the quality was reliable after installing the temperature controller. They said that the quality inspection would be checked before the factory.
On the black cat complaint platform, some consumers said that the number of recall products was huge, involving 180,000 beauty instruments, and he had doubts about whether the instrument after receiving the maintenance after the maintenance. " In addition, the current product risk factor is very high, and the problem of burns is extremely prone to, so he also wants to return the goods, but it is also rejected by the merchant.
In the future, the reporter called the official customer service in the recall of the Chupu Company for consultation. The first generation of the STOP EYE home frequency shot beauty instrument was safe and effective. At present Complaints about skin adverse reactions.
At present, the black cat complaint platform has nearly 100 complaints on the recallation of Stop Tripollar's first -Pu home radio frequency beauty instrument recall. There are as many as 165 users on the small red book platform.
Consumers of defective products have the right to choose to repair, replace or return the goods
According to the previous Beiqing Daily "Consumers of defective products have the right to return lawyers: can be reflected to the municipal supervision department", according to the provisions of the product quality law, consumers have the right to choose to repair, replace or return the defective products, have not eliminated defects or reduce safety Risks are not allowed to be sold or delivered again. If a producer causes personal or property damage to the defective products, it shall also bear the liability for compensation. Producers or sellers should not restrict the reasonable requirements of consumers. If consumers do not recognize the recall measures, they can report to the market supervision and management department.
According to Tianyancha, Tripollar, a well -known beauty instrument brand from Israel, was established in 2015 with its operating company's new foundation. The company's personnel were less than 50.
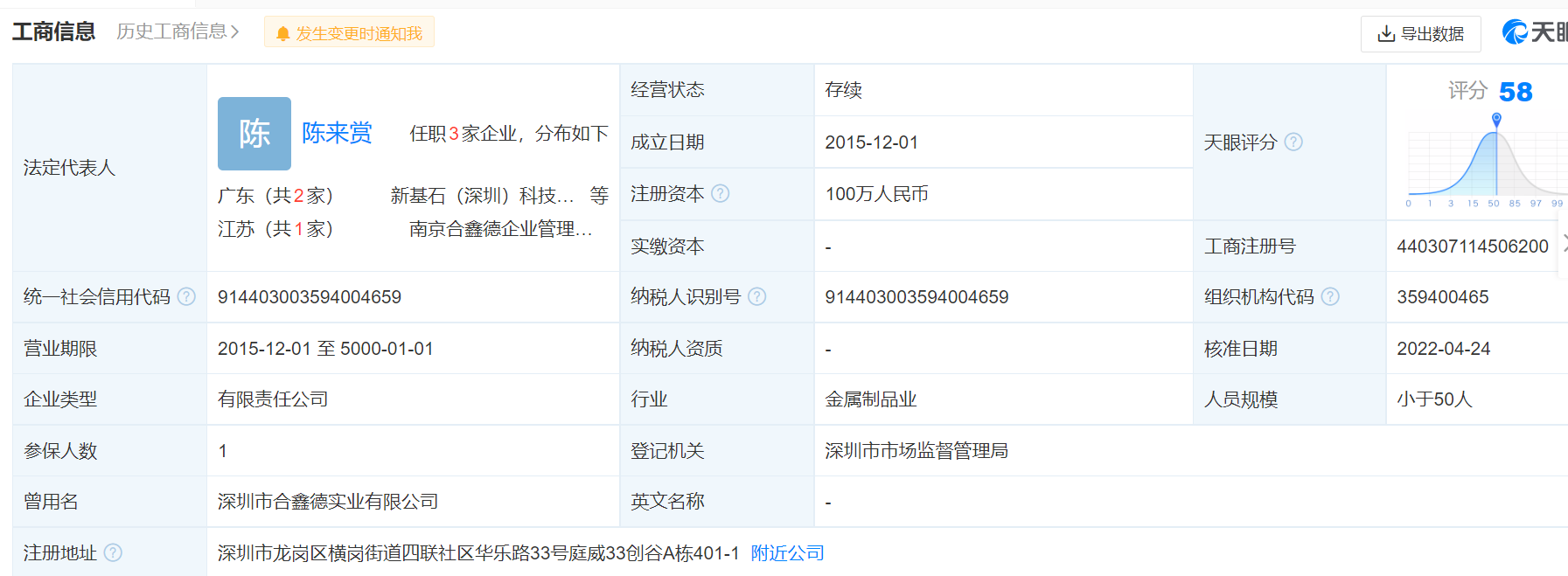
Picture source: Tianyancha
Well -known beauty instrument brands frequently roll over?
During the 3.15 International Consumer Rights Protection Day in 2021, China Grid and China Institute of Inspection and Quarantine Sciences jointly carried out the "Household Beauty Instrument Comparison Test" to temperature, electrical safety, etc. on the market on the market, etc. Testing. Among them, the first -generation STOP EYE beauty instrument test did not meet the standard. The assessment conclusion was that the temperature was up to 60 degrees Celsius during use, and there was a risk of burns. In this regard, the beginning of the Pu believes that "extreme testing" cannot be used as a basis for the security of RF beauty instrument.
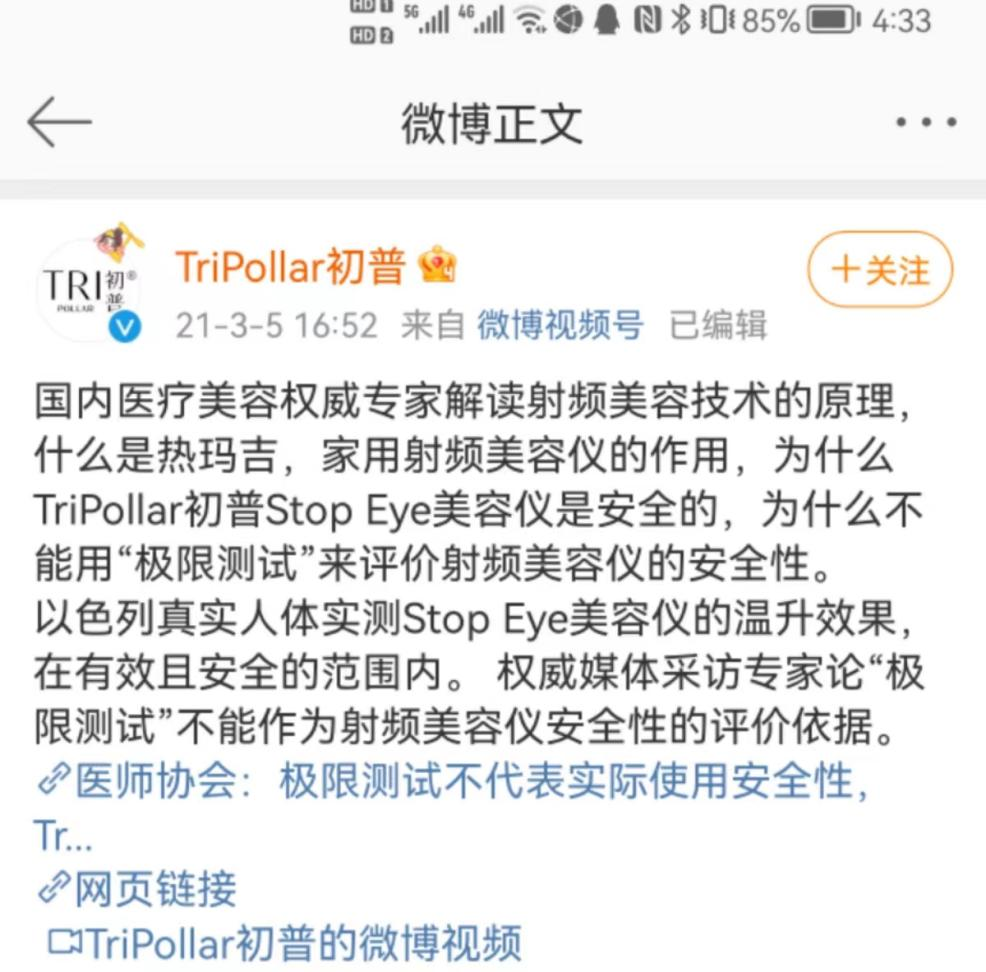
Picture source: Chupu official Weibo
In August 2021, Chu Pu appeared on Weibo for suspected false propaganda. Some consumers pointed out that the STOP VX and Stop Eye of the Chu Pu Beauty instrument used the "FDA Technology Certification" as a publicity selling point, but the "FDA technology certification" was not actually obtained. After the problem was fermented in large areas, the first Pu had characterized the matter into translation errors, and then withdrew the "FDA certification" of various sales platforms.
In fact, the relevant departments are also strengthening supervision in response to the current chaos of my country's beauty instrument industry.
On April 16, 2021, the State Drug Administration issued a notice of the "Principles of the Guidance Principles of the Classification Definition of RF Beauty Products" (Draft for Solicitation of Opinions). RF beauty products should be managed according to medical equipment. On March 30, 2022, the State Drug Administration issued the "Announcement on Adjusting the" Catalog of Medical Device Classification "(No. 30, 2022)" shows that the 09-07-02 radio frequency treatment involved in the attachment ( Non -disciplinary) Equipment device and radio frequency skin therapy instrument products in the device, from the date of this announcement, can apply for registration in accordance with the "Measures for the Registration of Medical Device Registration and Records" (State Administration of Market Supervision and Management No. 47) Essence Starting from April 1, 2024, radio frequency therapy and radio frequency skin therapy instrument products have not obtained medical device registration certificates in accordance with the law.
It can be said that the "Administrative Measures for the Registration and File of Medical Device" defines the radio frequency beauty instrument as "medical equipment", marking that the relevant departments are strengthening the supervision of the industry. As the strength of the supervision increases, the management of my country's beauty instrument industry will gradually gradually gradually gradually gradually gradually gradually gradually gradually gradually gradually gradually gradually. Towards standardization.
- END -
The rise of the ambition number one "Qiu Xiang 828"

In the summer of July, it was not yet bright. The Shenzhen Haiji Star Agricultural...
Grasp the party building strong branch to build a strong outposting fortress, resolutely win the epidemic prevention and control and resistance war

Grasp the party building strong branch to build a strong outposting fortress, reso...