[Caizhi Headline] Can the inhaled new crown vaccine lead Kangshino back to its peak?
Author:China Well -off Time:2022.09.07

Picture source: network
Kangshino (688185.SH) On September 5th, A shares+H shares opened high. As of the close of the day, A -share Kangxino opened 11.51%high and closed up by 9.82%; H shares Kangxino Bio opened up nearly 9%and closed up 7%.
It is worth noting that as the vaccine cow stocks that once held a group, Kangsino's stock price started from a high level and was already cut again. The trend of Hong Kong stocks Kangxino Bio-B (06185.HK) was similar. It was listed in Hong Kong stocks as early as 2019. In the past two years, the standard "roller coaster" trend.
Today, the stock price of Kangxino's stock price originated from the good news of the previous day. Kangxino announced that the company developed a new type of coronary virus vaccine (5 adenovirus vectors) for reorganization and proposed by the National Health and Health Commission, and the State Drug Administration organized a demonstration and agreed to enhance the needle into emergency use.

Photo source: Kangshino Announcement
This vaccine is also the same vaccine as the new crown vaccine "Kwaisa" that has been approved for listing. It uses a atomizer to atomize the vaccine into tiny particles and complete the vaccination by inhaling the oral cavity.
At the same time, Kangshino said that if the subsequent national departments are purchased and used in their procurement, it will have a certain positive impact on the performance of listed companies.
However, it should be noted that although clinical data shows that the price of the vaccine is relatively high, as the global new crown vaccine vaccination rate is saturated, the sales of products can drive for Kangshino's performance.
The world's first!
Public information shows that inhaling new coronary virus vaccines (5 adenovirus vectors) was approved by the National Drug Administration's drug clinical trial approval in March 2021. The indication was the disease caused by the prevention of new coronary virus infections (COVID-19) Essence
Experts said that inhaled vaccines may be an important supplement to the existing new crown vaccine. Compared with muscle injection vaccines, inhaled vaccines are expected to provide more protection in the "first line of defense" of the virus entering the human body through the respiratory mucosal immune response to the "first line of defense" of the virus entering the human body.
In November last year, Kangshino's official website issued a press release of the "Kangshino Biotechnology inhaled new crown vaccine", which made a detailed explanation of the vaccination method and mechanism of the vaccine. The inhaled new crown vaccine needs to be atomized first, and it is handed over to the vaccinator as soon as possible after the atomization is completed. The inhalation method of inoculating the inhalation of the lungs in order is to exhale the lung gas, hold the cup mouth, suck the gas in the cup within 10 seconds, and hold the breathing for 5 seconds.
From the perspective of the mechanism of action, the new coronary virus invades the body by infection with the respiratory mucosal epithelial cells. The mucosal immune system is the first immune line of the body. Establishing a good mucosa immunity can be killed before the new crown virus invades tissue to protect the body tissue of the body tissue. For exemption. The results of the study show that the antibody produced by the inhaled new crown vaccine in the mucous membrane is earlier than the serum antibody, the cost is high and the maintenance time is longer.
According to the half -annual report of Kangxino in 2022, the total investment scale of the reorganized new coronary virus vaccine is 300 million yuan, and the cumulative investment amount is 95.502 million yuan.
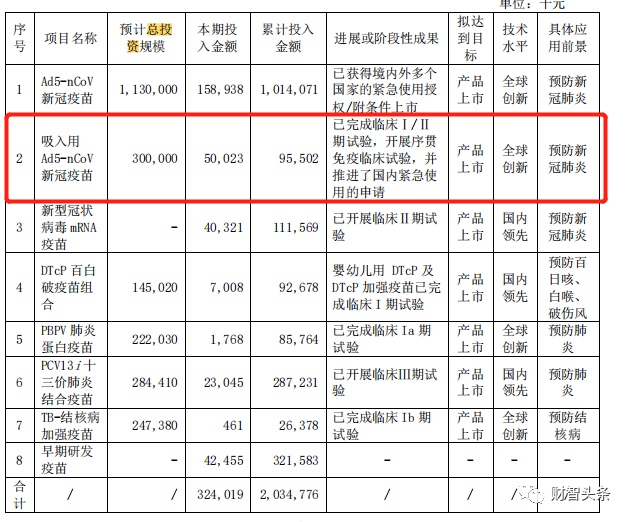
Photo source: Kangshino's half -annual report in 2022
In this regard, Guojin Securities Research Report pointed out that the inhalation dosage form was approved, which enriched the commercial layout of Kangxino on the new crown vaccine. In the future, there will be a possibility of further acquisition of Chinese government procurement and the future entry of WHO procurement lists.
However, this does not mean that Kang Xino can "care".
Before the vaccine, the vaccine needs to apply for clinical trials, conduct clinical trials, apply for production number, and product approval. The follow -up R & D and administrative approval of the reorganized new coronary virus vaccine (type 5 gonad virus vector) still has certain uncertainty.
According to Kangxino's announcement, after inquiries, there have been 9 vaccines in China that have been approved by the State Drug Administration to be listed or urgently used in the state of the state drug supervision. stage.
According to statistics, in addition to the reorganization of new coronary virus vaccines with reorganization, 8 other domestic vaccines in China have been approved for listing or urgently used vaccines in China, namely: Chinese creature-northern survival vaccine, Chinese biological-Wuhan surviving Vaccine, Kochinzhong Vaccine, Kangxino adenovirus vector vaccine, Zhifei Bio -restructuring protein vaccine, Kangtai biochemical vaccine, the surplus vaccine of the medical sciences, and the new coronary virus fusion protein vaccine of Lizhu Group.
In the announcement, Kangxino also prompted relevant risks, saying that even if the product is included in emergency use, its future market sales will still face a fierce competition, and at the same time, the development and changes of the domestic and foreign epidemic conditions, the domestic new crown vaccination vaccination Affected by various factors such as rates.
What is the effect of inhaling new crown vaccines as a "enhanced needle"?
In October last year, Zhu Tao, chief scientific officer of Kangshino Bio, publicly stated that it can be enhanced by heterogeneous sequences with inhalation of the new crown vaccine of adenovirus vectors, which can induce high -level IgG antibodies and cellular immune responses. The rise of 250-300 times is more advantageous compared to the use of the same vaccine in the third needle.

Picture source: Kangshino Vaccine Public Account
According to the study published in the international topic journal "Willow Knife" in May this year, Kwaisa Wusi not only stimulates body fluid immunity and cellular immunity, but also efficiently induce mucosal immunity, achieve triple protection, block infection and spread.
Immunosity results show that after 28 days of inhalation with Kwaisa sequence, the two dose groups for the neutral antibody levels of the original strain were 18.4-26.4 times the exactly the same -source reinforcement group (low -dose GMT GMT GMT : 1937.3, high -dose GMT: 1350.8, and the active homologous enhanced group GMT: 73.5). At the same time, the orderly strengthening of the inhalation vaccine also has high -level cross protection to Delta mutant strains. The neutralized antibody level is 18.1-24 times that of the activated vaccine. Can the inhaled new crown vaccine bring Feikangxino?
Kangshino is an innovative vaccine company. Its main product is reorganized new coronary virus vaccine (type 5 adenovirus carrier), meningitis vaccine vaccine, restructuring Ebola virus vaccine (adenovirus carrier), thunderous vaccine, pneumonia, pneumonia Bacteria combined with vaccines, tuberculosis, and reinforcement vaccines.
In March 2019, Kangshino was listed on the main board of the Hong Kong Stock Exchange; on August 13, 2020, it officially landed on the Science and Technology Board, becoming the first "A+H" vaccine stock since the opening of the science and technology innovation board.
Among its listed products, the launch of the new coronary virus vaccine (type 5 gonad virus carrier) Kwaisa has successfully reversed the situation of Cumino's many years of losses. Data show that the vaccine and Chen Wei team of the Chinese Academy of Military Sciences of the Chinese People's Liberation Army jointly developed and were launched in February 2021.
Data show that from 2016 to 2020, Kangsino's net profit losses in his mother increased year by year, with losses of 50 million yuan, 64 million yuan, 138 million yuan, 157 million yuan, and 397 million yuan, a total loss of 806 million yuan.
By 2021, Kangshino's new crown vaccine Kwaisa was approved to go public, and from February, it has obtained emergency use authorization/attached conditions in many overseas countries. Thanks to this, Kangshino's operating performance has soared. In 2021, operating income was 4.3 billion yuan, an increase of 17175%year -on -year; net profit was 1.914 billion yuan, an increase of 582.65%year -on -year; %.
However, this high -speed growth trend did not last long. In the first half of 2022, Kangshino's revenue fell by 69.45%year -on -year to 630 million yuan, and its net profit was 12.238 million yuan, a year -on -year decrease of 98.69%.
Picture source: Kangshino 2022 Semi -annual report
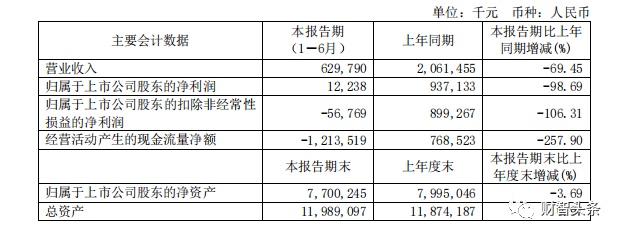
Blessing, no scourge, with the release of the performance of Kangxino's interim report, its stock price fell. On August 29, Kangshino closed more than 15%to close at 130.6 yuan/stock. In the past year, Cumano's stock price has fallen by 63.5%.
You know, in 2020, the issue price of Kangshino's issue at the science and technology board was 209.71 yuan/share, second only to Stone Technology, or the second highest issue price in A -share history.
Nowadays, as the inhalation of new coronary virus vaccines is approved for emergency use, today's stock price has been adjusted. In the long run, can the inhalation reorganized new coronary virus vaccine led Cumino to return to its peak?
New crown vaccination rate is becoming more saturated
Market space needs to be observed
To what extent can the inhaling new crown vaccine drive the company's performance?
According to the CCTV report last April, Yulianfeng, Chairman of Kangxino, said that the company's new crown vaccine capacity is gradually getting measured. The annual capacity of 500 million doses of a single needle vaccine is based. The annual capacity of the new crown vaccine may reach 2.5 billion doses in the future.
It is worth noting that compared with previous years, the overall operating income of vaccine companies in the first half of 2022 declined, and net profit increased negatively. Taking Kangxino as an example, benefiting from the global new crown vaccination, its income and net profit attributable to the mother of 2021 achieved explosive growth.
In February of this year, Kangshino's injected new coronary virus vaccine (type 5 gonad virus carrier) was approved to be used to enhance immunization, but it had a small effect on performance pull. As the domestic new crown vaccination rate is close to saturation, Kangxino's revenue and net profit drop.
In addition to the inhalation of the oral cavity, the new crown vaccine inhaled in the nasal cavity can also directly act on the respiratory tract. At present, Wantai Biological (603392.SH) and China Pharmaceutical Group Co., Ltd. China Biotechnology Co., Ltd. is actively deploying the nasal suction new crown vaccine products.
According to Wantai Biological Corporation, the calculated sales price of the first product of the first product of the newly -crown -nasal nasal injection vaccine of the nasal injection vaccine industry base was 50 yuan/branch (including tax). At present, Conchino has not announced information such as product prices and commercial plans.
According to the data, Wantai Biological Corporation's fixed increase plan is expected to have a total investment of about 1 billion yuan in the total investment of the nasal injection vaccine industry. It is planned to be completed in 2023, and the production capacity can reach 240 million doses/year.
At the same time, the business story of the new crown vaccine continues. According to sorting out the August Report of Southwest Securities, as of August 26, there have been new crown vaccines from 7 domestic companies that can be sold in China, but more than 20 more models have been developing vaccines to sprint.
(WeChat public account "Caizhi Headline" comprehensive self -consuming: Yangguang.com, Finance Association, Daily Economic News, Interface News, etc.)
Edit: Bai Jing
School pair: Yuan Kai
Review: Gong Zimo
- END -
Anti -epidemic photo: Financial "Daibai" to enter the village on -site service

Anti -epidemic photo: Financial Daibai to enter the village on -site serviceTian J...
"I call on cross -border e -commerce companies across the country to come to Ningbo!"

Live. Photo by reporter Ke ShanluI call on cross -border e -commerce companies acr...