Core products Huoxiangzhengqi water methanol methanol testing is not compliant, Tai Chi Group's low R & D high -sales high sales argument
Author:Blue Whale Finance Time:2022.09.02

Huoxiangzhengqishui, as a highly well -known medium -propelled medicine, is widely known as Tai Chi Group, but on August 29, the State Drug Administration's "National Drug Administration on 20 batches of drugs did not meet the requirements of the regulations. The notice (No. 39, 2022) shows that the Huoxiang Zhengqi (batch number: 2021013, 2021046) produced by Tai Chi Group Sichuan Nanchong Pharmaceutical Co., Ltd. does not meet the regulations.
As soon as the news came out, it caused a heated discussion. On the evening of August 30, Tai Chi Group issued an announcement to respond to this: the unsweight part has been recalled. However, this is not the first time that Tai Chi Group has experienced product quality problems. In addition, as an enterprise with a revenue of over 10 billion, the low R & D expenses are low, and the imbalance with sales costs has always been a common concern in the industry.
The quality of the old -fashioned product is frequent problems
Public information shows that Tai Chi Group is mainly engaged in the processing and sales of Chinese medicine, western medicine, health products, processing of medical packaging products, and medical device sales. Its main products are Chinese medicine products such as Huoxiangzhengqi Oral solution, urgent syrup, and Tongtian oral solution.
Among them, Huoxiang Zhengqi Oral Oral Liquid is the core product of over 1 billion sales of Tai Chi Group. Tai Chi Group's 2022 semi -annual report shows that in the first half of the year, Huoxiangzhengqi Oral Oral solution achieved sales revenue of 998 million yuan, an increase of 79%year -on -year. The company's total revenue of 4.549 billion yuan in the total pharmaceutical industry revenue.
Interestingly, the "Huoxiangzhengqi Ice Chinese" trend has been scratched on the Internet, and it is about to drink in water with ice cubes such as Huoxiangzheng Qiqi and Huoxiang Zhengqi. Coffee, want to neutralize the taste of coffee and Huoxiang Qiqi liquid at the same time to achieve the role of "wake up" and "clearing heat", which causes disputes on safe medication.
In this regard, a number of medical experts reminded in an interview with the media that Huoxiang Zhengqi liquid belongs to over -the -counter drugs and is used for disease treatment and cannot be taken as a drink for drinking.
Huoxiangzhengqi water, which occurs this time, is often confused with Huoxiang Zhengqi oral liquid. However, the biggest difference between the two is that Huoxiangzhengqi water contains ethanol, while Huoxiang Zhengqi oral liquid does not contain ethanol. Huoxiangzhengqi Water's method is to use 60%ethanol for solvents with 60%ethanol, filtering after impretion, and mixing the filter fluid and mixing. The Huoxiang Zhengqi oral liquid system is to remove the medicinal material with 60%ethanol, and then remove the ethanol in the extract solution, and only keep the aqueous solution.
The detection of the amount of methanol methanol in Huoxiangzhengqi does not meet the regulations. According to the explanation of the State Drug Administration, in the test, the methanol volume reflects the methanol content brought by ethanol in ethanol preparations such as alcohol.
Zhang Xusheng, a toxicist of the Chinese Society, said in an interview with Times Finance that generally there may be adulteration of alcoholic beverages in food, which refers to the use of methanol (industrial alcohol) instead of ethanol to cause food poisoning. (The amount of methanol in alcoholic medicine is not in charge.) This situation may involve alcohol adulteration, or the purification process of alcohol is not perfect, which contains a small amount of methanol, which depends on the test results to judge.
Public information shows that methanol and ethanol (drinking wine) have a very similar odor, but different from ethanol, which is high to toxic methanol and cannot be consumed. Drinking more than 4 ml of poisoning will occur, and more than 10 ml can cause blindness due to permanent destruction of the optic nerve. 30 ml can cause death.
Regarding the incident, Tai Chi Group announced on August 30: Based on the principles of responsibility for consumers, Nanchong Pharmaceutical's 15677 boxes of the above -mentioned batch number products have been recalled according to the procedure. The company apologized sincerely about the adverse impact caused by the incident. In addition, Tai Chi Group's statement will be carefully rectified to ensure that the quality of the product is qualified.
However, this is not the first time that Tai Chi Group has experienced product quality problems.
On July 29, the drug spot inspection and inspection information released by the Guangdong Pharmaceutical Supervision Bureau showed that the penicillin V potassium tablets (batch number: 2011103) produced by the Tai Chi Group Holding subsidiary Southwest Pharmaceutical Co., Ltd. was unqualified.
In July 2020, the website of the Hainan Provincial Drug Administration released "About the 7th issue of 2020 (Total Fifty -eighth) Pharmaceutical Sample Inspection Inspection Information Notice" shows that the 2 batches of two companies including Tai Chi Group Sichuan Mianyang Pharmaceutical Co., Ltd. Sub -drug random inspection is unqualified.
Tracing back forward, in 2016, its "pediatric cough and asthma particles" was identified as inferior drugs after being sampled; the same year, the orange red pill was detected by a higher sulfur content; Unqualified products; in 2020, "Tongxuanli Pills" was sampling as unqualified products.
MSI sales are not as expected, and the cost of R & D and sales is imbalanced
Regarding the impact of non -compliance incidents on methanol testing on the company, Tai Chi Group stated that among the company and subordinate enterprises, only Nanchong Pharmaceutical production and sales. 6.65 million yuan, accounting for 0.05%of the company's operating income in 2021; this incident involved product sales revenue of about 220,000 yuan, accounting for a small proportion of the company's operating income, and has no significant impact on the company's production and operation.
In fact, compared to Huoxiang Zhengqi, which is smaller, Huoxiang Zhengqi related products of Tai Chi Group also include Huoxiang Zhengqi Oral Oral Liquid, Huoxiangzhengqi Granules, Huoxiangzhengqi Capsules, Huoxiang Zhengqi Pills, etc. According to the data of Zhongkang CHIS Kaishi System, Tai Chi Group's Huoxiang Zhengqi series products were as high as 58%in 2021, especially the total proportion of retail channels was as high as 92%. Among them, Huoxiang Zhengqi Oral Liquid is the fist of Tai Chi Group. Tai Chi Group once formulated a ten -year strategic plan for Huoxiang Zhengqi Oral Oral Liquid, that is, domestic sales reached 2 billion yuan in 2018, 5 billion yuan in 2021, and 10 billion yuan in sales in 2027.
However, in recent years, the sales of Huoxiang Zhengqi oral liquid have not reached the vision of its "10 billion items". In 2017, the sales of Huoxiang Zhengqi oral liquid began to decline after the peak of 1.35 billion yuan. From 2019 to 2021, the sales of the single product were 600 million yuan, 689 million yuan and 920 million yuan.
In addition to the unpredictable sales of single products, for a company with a revenue of more than 10 billion yuan, the low R & D expenses have been low, and the imbalance with sales costs has always been a common concern in the industry.
From the perspective of R & D investment, the proportion of R & D investment expenditures in Tai Chi Group has always been low. The financial report shows that in the five years from 2017 to 2021, the R & D expenses of Tai Chi Group were 33.5668 million yuan, 420.824 million yuan, 67.1749 million yuan, 96.5087 million yuan, and 103 million yuan, and the R & D cost rates were not more than 1%.
Compared with R & D expenses, it is a rapid growth in sales expenses. From 2017 to 2021, the sales expenses of Tai Chi Group were 1.908 billion yuan, 2.894 billion yuan, 3.751 billion yuan, 3.701 billion yuan and 4.215 billion yuan, with a cumulative 16.469 billion yuan in five years.
High amount of sales costs have not brought net profit growth to Tai Chi Group. The annual report shows that between 2017 and 2021, the revenue of Tai Chi Group increased from 8.735 billion yuan to 12.149 billion yuan; but the net profit of the deduction of non-returnees was only 64.3748 million yuan, -83.6294 million yuan, respectively. 157 million yuan, -556 million yuan, -697 million yuan.
According to the semi -annual report released recently, in the first half of this year, Tai Chi Group achieved operating income of 7.185 billion yuan, an increase of 11.52%year -on -year; the net profit attributable to shareholders of listed companies was 124 million yuan, an increase of 61.40%year -on -year; The company's shareholders' net profit was 179 million yuan, an increase of 112.48%.
Regarding revenue growth, Tai Chi Group stated that it is mainly due to the growth of core products such as Huoxiangzhengqi Oral Oral solution. However, it is worth noting that its sales cost of the first half of the year reached 2.444 billion, an increase of 21.45%compared with the same period last year; R & D expenses, 44.652 million, an increase of 7.52%compared to the same period last year. The current status of light sales has not changed.
- END -
The main preparation work of the 7th China -Asia -Europe Expo is basically completed
Tianshan News (Reporter Wang Yongfei) As of August 14, the preparations for the opening ceremony of the seventh China -Asia -Europe Expo, forum, and trade promotion activities have been basically comp
Meitu Company's mid -term performance in 2022: Monthly active users of 240.9 million increased by 4.5 % from the previous month
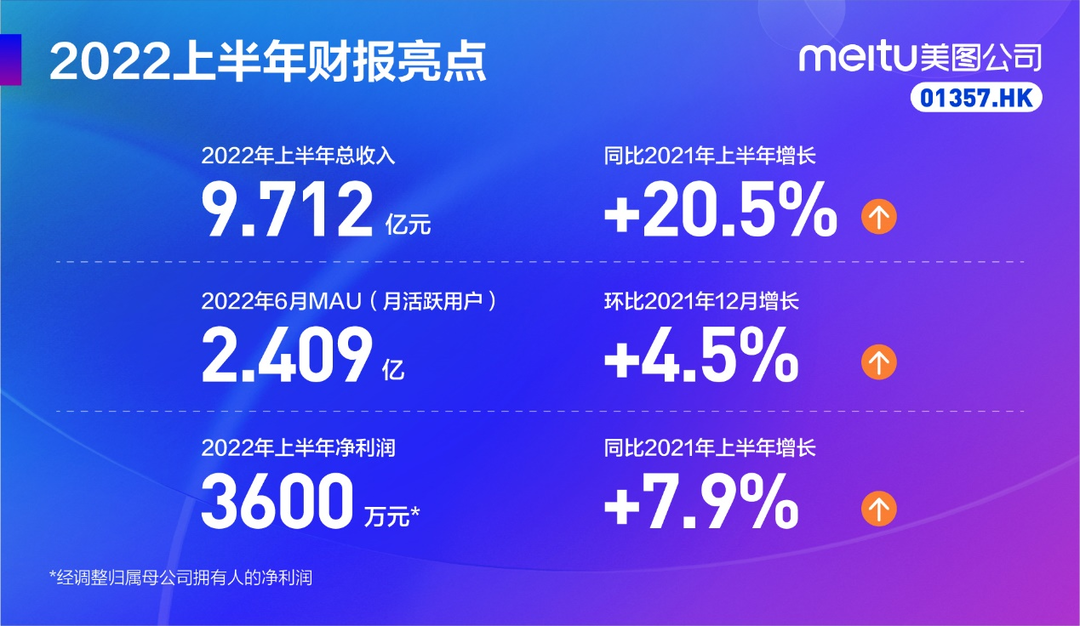
Cover news reporter Zhang YuexiOn August 31, Meitu officially disclosed the 2022 m...