The clinical mass spectrum enters the index level growth inflection point, Maldi-TOF is the treasure market that has been ignored
Author:Arterial network Time:2022.08.25
How hot is the Chinese clinical mass spectrometry? Financing data can give people the most intuitive feelings.
According to the statistics of the arterial network, there are currently 80 financing incidents in the clinical mass spectrometer industry, with a total of more than 2.7 billion financing quotas, and existing enterprises have entered the stage of round D financing. The number of financing incidents in 2021 reached 17, and the annual financing volume exceeded 1.15 billion yuan, the highest in the past 10 years. Star investment institutions such as Gao Yan Venture Capital, IDG Capital, Junlian Capital, Shilba Investment, and Jingwei China also bet on betting, showing confidence in the track.
Especially in 2022, the clinical mass spectrum is further received by the capital market. Since 2022, a total of 13 financing incidents have occurred in the clinical mass spectrum, with a total amount of more than 900 million yuan. It is estimated that in 2022, the number and amount of clinical mass spectrometers will be innovative.

Clinical quality spectrum financing situation over the years
The clinical mass spectrum has entered the inflection point of the index level, and companies in the upstream and downstream of the industrial chain have also accelerated the pace of development. How can I calm down the industry's next growth opportunity in a high heat? The arterial network believes that the future opportunities of the industry are hidden in the direction of MALDI-TOF, localization of instruments, and improvement of operating efficiency. We have analyzed the dimensions of instruments, technology platforms, and operating efficiency.
MALDI-TOF space exceeds 100 billion, nucleic acid mass spectrum is new forces
The clinical mass spectrum does not only refer to a technical platform, but consists of series spectrum, MALDI TOF, ICP-MS multiple technology platforms. Different technical platforms have their own characteristics and can achieve precise testing of various logo.
From the characteristics of various technical platforms, the number of sectors in series is good, sensitive and specific, and are suitable for detecting small molecules. Applications in scenarios such as newborns screening, drug concentration monitoring, vitamin detection are increasing rapidly.
Maldi-TOF has great application potential that has not been tapped. The application of MALDI-TOF in microbial identification is relatively mature. In fact, Maldi-TOF has a large molecular weight range. It can detect macromolecules and small molecules. The application space in nucleic acid detection, mass spectrometry, and protein quantitative detection is very large.

Summary of the characteristics of different technical platforms
According to the data provided by Yixin Quality spectrum, Maldi-TOF's market space exceeds 100 billion yuan, of which the market size of microorganisms is about 2 billion, and the drug genome (including mental, tumor, hypertension, hyperlipidemia, anticoagulant anesthesia and other drugs ) About 40-50 billion yuan, genetic disease testing (including deaf genes, thalassemia, etc.) between 5 and 8 billion, infectious disease testing is about 20 billion.

MALDI TOF molecular weight range is the largest, there are many detected items, and the corresponding market space is even larger.
In the clinical mass spectrometry, nucleic acid mass spectrum is the next important growth point.
The PCR flux is low. It can only detect several sites. NGS is large, and tens of thousands of sites can be detected, but the cost is very high. The number of sites of drug genome detection is generally between dozens to two hundred or two hundred. Deaf genes and thalassemia need to be tested more than 20 sites. PCR detection flux is insufficient. The cost of NGS is too high, and the detection time is too long. Nucleic acid mass spectrometry is low, fast cycle, and simple operation, filling the gap of the medium -to -average detection.
More and more companies have gathered their attention to nucleic acid mass spectrometry, and Yixin mass spectrometry has taken the lead in crossing the threshold for approval of nucleic acid mass spectrometers.
In 2016, the CLIN-TOF developed by Yixin Quality spectrum was approved for nucleic acid detection, which achieved simultaneous microbial identification and nucleic acid testing. At present, Clin-TOF has been widely used in pharmaceutical genome testing, genetic genetic testing, and epidemiological testing. The Yixin Quality spectrum also participated in the formulation of the first Chinese nucleic acid mass spectrometry.
At present, in addition to the approval threshold of the instrument, the threshold of the nucleic acid mass spectrum also includes chips, reagents and related software databases. Yixin Quality spectrum has fully opened up the development path of nucleic acid mass spectrometry instruments, chips, reagents, and software databases, published a number of papers, and has a number of patents.
In 2015, Yixin Quality spectrum and Shimadzu reached cooperation on nucleic acid mass spectrums. At present, the cooperation has lasted for 7 years. In addition, just recently, the China Centers for Disease Control and Control Center Virus and Control and Control Institute has evaluated the effectiveness of Yinxin Quality Spectrum based on micro-array chips-flight time mass spectrometry method to detect the effectiveness of the detection of multi-testing kit detection of the test box detection, showing that the product Has high accuracy, consistency and repetitiveness.
In the future, nucleic acid mass spectrometry proof also needs long -term investment and large -scale capital investment. With the increase of nucleic acid mass spectrometry companies and reagents approve, the nucleic acid mass spectrum will drive the clinical mass spectrometry industry to enter the next period of explosive growth.
Only localized instrument can release the clinical mass spectrum hundreds of billions of blue sea
At present, the clinical mass spectrometry has been deployed by enterprises.

The layout of domestic and foreign companies on various technical platforms of clinical mass spectrometry
The upstream of the clinical mass spectrometry is similar to the gene sequencing industry. Due to the difficulty of localization of upstream instruments, the hotness of the clinical mass spectrometry is the first to be reflected in the detection application.
The localization of instruments requires long-term R & D investment. Generally spending 5-10 years, enterprises are focused on the inspection application side, which can quickly push the product and services to the market, generate cash flow, and ensure the rapid growth of the enterprise. Based on the characteristics of the industry, early detection of the application side seeks breakthroughs, and the clinical education is mature. After the market is opened, the development of domestic instruments will be developed to achieve domestic alternatives. It is a common practice for the industry, especially LC-MS companies. Therefore, at this stage, in terms of financing performance, the detection of enterprises on the application side are even more eye -catching and have obtained a large amount of financing from many investment institutions. There are fewer companies focusing on localization of instruments, only Yixin Quality spectrum and national medicine industry.
Now there are many companies that lay out clinical mass spectrometry detection applications. Normally test applications such as newborn screening, vitamin detection, and drug concentration monitoring have already been approved by products. Also increasing.
In the early stage, mass spectrometer detection and application promoted the explosive growth of China's clinical mass spectrometer industry. Now, as the regular application registration certificate continues to increase, the detection application side is increasingly crowded, and the domestic marketing market of upstream instruments has not yet been fully developed, and the opportunities are huge. Driven by domestic alternative factors, it has reached the time when the upstream instrument is localized.
The localization of instruments is the direction of policy encouragement. In 2021, the "Guidance Standards for Government Procurement and Imported Products" jointly issued by the Ministry of Finance and the Ministry of Industry and Information Technology clearly stipulates the proportion requirements of government institutions (institutions) to procure domestic medical devices and instruments. The procurement rate of domestic brands of high -efficiency liquid chromatography couplets is at least 25%.
It is clear that the domesticization of instruments is an inevitable choice of the industry. The healthy development of the industry needs long -term vision. The enterprise needs to sink to the development of domestic instruments.
In the early days, relying on testing applications, the clinical mass spectrum market ran fast. At present, the market size has reached billions of, but it must not be lacking in the help of the instrument ’s domesticization for a long time. Market blue ocean.
At present, the localization of mass spectrometry instruments is still in early start.
In terms of MALDI-TOF and ICP-MS, Yixin Quality spectrum, as the earliest group of clinical mass spectrometers in China, its independently developed Clin-TOF is the first approved MALDI-TOF in China. Clin-ICP-QMS is the first domestic ICP-MS approved.
Among them, patents are the key to clinical mass spectrometer corners. In the Maldi-TOF patent, Brook, Merrier, and Agare are the core technology owners of Maldi-TOF. The layout of Yixin Quality spectrum patent has maintained synchronization with foreign applicants. The patent has applied for more than 200 applications, and has been authorized by more than 60 items.
At present, the number of quality spectrometers developed by Yixin Quality spectrum has exceeded 100 units, covering more than 60 well -known medical institutions and testing centers in China, such as Beijing Union Hospital, 301 Hospital, Zhongshan Hospital, China Centers for Disease Control and Prevention, and Huada Gene. Leading in China.
The localization of instruments is an important part of building China's leading clinical mass spectrometry ecosystem. Next, the attention of enterprises and investors' attention to the localization of mass spectrometers will rise rapidly.
Clinical mass spectrometry, operating efficiency is the core
The clinical mass spectrum is a 100 billion -level market, and its potential to be tapped is expected to exceed gene sequencing. With the spraying of the clinical mass spectrometer industry in the past two years and the acceleration of enterprises, many enterprises have performed amazing financing capabilities, research and development capabilities, and marketing capabilities.
The financing capabilities are the underlying help of R & D and marketing. R & D capabilities determine the ceiling of the enterprise. How big the market can enter and the marketing capabilities determine. However, there are such situations in all walks of life. The financing capabilities are far ahead, but they have not created the value of applications. The research and development strength is strong, and the commercialization of the product is not ideal. In addition to financing capabilities, research and development capabilities, and marketing capabilities, it is more important to pay attention to the input -output ratio.
Yixin Quality spectrum has 19 years of experience in the field of mass spectrometry. The technical products are excellent, the output has exceeded 100 units, and the cost of the unit has gradually entered the smooth period, and the scale advantage is outstanding. At the same time, the company has accumulated a number of customers of many three hospitals, with an income of 27 million yuan in 2021, of which sales investment accounts for only 10%, and the operating efficiency is much higher than the industry average. In June 2022, Yixin Quality spectrum and Kang Sheng Global reached the cooperation of the entire product line.
As the output increase, the cost of the unit gradually decreases, and the quality gradually increases

In the future, the strategic position of mass production instruments will become more prominent. Maldi-TOF will become one of the mainstream technology platforms. New opportunities for clinical mass spectrometry include localization of instruments and nucleic acid mass spectrometry. From detection and application to the localization of instruments, from microbial material spectrum to nucleic acid mass spectrometry, this process will eliminate a large number of enterprises, key competition elements among enterprises will be concentrated in financing capabilities, research and development capabilities, and marketing capabilities. Enterprises need to make breakthroughs in operating efficiency. Essence
- END -
U.S. stocks closed: Three major indexes rose four consecutive surrounding five companies to retire from the municipal Chinese enterprises collectively closed down
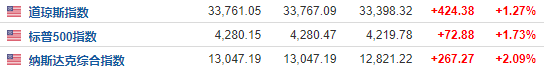
U.S. stocks opened high on Friday (August 12), and the three major indexes increas...
Qian -acres of oil sunflower in Xintao Township, Gulang County: Beautiful in the countryside, rich in the villagers

In recent years, Xinbao Township, Gulang County has adhered to the development goa...